| Tulathromycin A |
| (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[[2,6-Dideoxy-3-C-methyl-3-O-methyl-4-C-[(propylamino)methyl]-a-L-ribo-hexopyranosyl]oxy]-2-ethyl-3,4,10-trihydroxy-3,5,8,10,12,14-hexamethyl-11-[[3,4,6-trideox |
| (CAS 217500-96-4) |
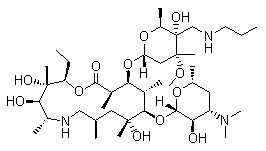 |
| Description: |
Tulathromycin A is a macrolide antibiotic.
IC50 Value: 1 microg/ml (MIC90 for Pasteurella multocida)
Target: Antibacterial
in vitro: Two highly pathogenic strains of M. bovis (with minimum inhibitory concentration values for tulathromycin of 1 and >64 microg/ml) were inoculated into 145 calves. Four days after inoculation, calves with clinical BRD were treated subcutaneously with saline or tulathromycin (2.5 mg/kg). Compared with saline, BRD-related withdrawals, peak rectal temperatures, and lung lesion scores were significantly lower for tulathromycin-treated calves (P < .01). Tulathromycin was highly effective in the treatment of BRD due to M. bovis in calves regardless of the minimum inhibitory concentration of the challenge strain (1 or >64 microg/ml). The lowest concentrations inhibiting the growth of 90% of isolates (MIC90) for tulathromycin were 2 microg/ml for Mannheimia (Pasteurella) haemolytica, 1 microg/ml for Pasteurella multocida (bovine), and 2 microg/ml for Pasteurella multocida (porcine) and ranged from 0.5 to 4 microg/ml for Histophilus somni (Haemophilus somnus) and from 4 to 16 microg/ml for Actinobacillus pleuropneumoniae.
in vivo: Each study randomly allocated 250 calves to receivetulathromycin at 2.5 mg/kg and 250 calves to receive either tilmicosin at 10 mg/kg (Colorado site) or florfenicol at 40 mg/kg (Idaho and Texas sites) on arrival at the feedlot. Calves were housed by treatment group in pens with 50 calves/pen. The treatment groups were physiologic saline (n = 160) given SC at 0.02 ml/kg, tulathromycin (n = 320) given SC at 2.5 mg/kg, and tilmicosin (n = 320) given SC at 10 mg/kg .Tulathromycin is a triamilide antimicrobial that has been approved for use in the treatment and prevention of bovine respiratory disease and the treatment of swine respiratory disease.
Toxicity: No adverse events related to tulathromycin were reported.
Clinical trial:
|
| Product No. |
KT20139 |
| Product Name |
Tulathromycin A |
| Synonyms |
Tulathromycin; Draxxin; CP 472295 |
| Formal Name |
(2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[[2,6-Dideoxy-3-C-methyl-3-O-methyl-4-C-[(propylamino)methyl]-a-L-ribo-hexopyranosyl]oxy]-2-ethyl-3,4,10-trihydroxy-3,5,8,10,12,14-hexamethyl-11-[[3,4,6-trideox |
| CAS Number |
217500-96-4 |
| Molecular Formula |
C41H79N3O12 |
| Formula Weight |
806.08 |
| Formulation |
A crystalline solid |
| Purity |
98%min |
| Stability |
2 years |
| Storage |
-20°C |
| Shipping |
USD45 for Europe and USA. No shipping charge once amount reach USD500 |
| Quality Control |
HNMR,CNMR,LCMS,HPLC,IR,etc. |
| Price & Availability |
In Stock. Price Negotiated. |
|
| Related Products: |
Dibekacin sulphate
Arbekacin
Minocycline Hcl
Iguratimod
Daptomycin
Mitomycin C
Luliconazole
Valsartan
Tolvaptan
Prasugrel
Sitagliptin phosphate monohydrate
Tirofiban hydrochloride monohydrate
Aliskiren
Aliskiren hemifumarate
Nebivolol Hydrochloride
Orlistat
|
|


