| Carboplatin |
|
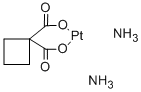
| (CAS 41575-94-4) |
 |
| Description: |
Carboplatin is a chemotherapy drug by binding to DNA and interfering with the cell's repair mechanism.
IC50 Value:
Target: DNA crosslinker
in vitro: Carboplatin exhibits an inhibitory effect on cell proliferation in a human ovarian cancer cell line panel, including A2780, SKOV3, and IGROV-1 cells with IC50 of 6.1 μM, 12.4 μM and 2.2 μM, respectively [1]. Carboplatin also show the anti-proliferative activities in lung carcinoid cell line, such as UMC-11, H727, and H835 cells with IC50 of 36.4 μM, 3.4 μM and 35.8 μM, respectively [2].
in vivo: In A2780 tumor xenografts, Carboplatin (60 mg/kg via i.p.) given as single agents shows a modest antitumor effect with the relative tumor volumes on day 6 of 8.4 compared to the control of 11.9, and the day 6 tumor weights relative to control (T/C) of 67% [1]. For the VC8 (Brca2-deficient) xenografts, Carboplatin treatment delays tumor growth and reduces tumor mass by 42% compared to the vehicle group [3].
|
| Product No. |
KT20792 |
| Product Name |
Carboplatin |
| Synonyms |
|
| Formal Name |
|
| CAS Number |
41575-94-4 |
| Molecular Formula |
C6H12N2O4Pt |
| Formula Weight |
371.25 |
| Formulation |
A crystalline solid |
| Purity |
98%min |
| Stability |
2 years |
| Storage |
-20°C |
| Shipping |
USD45 for Europe and USA. No shipping charge once amount reach USD500 |
| Quality Control |
HNMR,CNMR,LCMS,HPLC,IR,etc. |
| Price & Availability |
In Stock. Price Negotiated. |
|
| Related Products: |
Altretamine
Altretamine is an alkylating agent proposed as an antineoplastic.
Target: DNA alkylator
Altretamine represents a useful alternative in patients who prefer oral treatment or when socioeconomic considerations are an important issue. Altretamine is an acceptable and apparently less toxic alternative to other cytotoxic drugs used for palliation of patients with recurrent epithelial ovarian cancer. The inter-hand intrapatient variability of the bioavailability of altretamine after oral administration represents an important drawback for effective clinical use of this drug. The variability appears to be mostly related to the first-pass effect and therefore may be overcome by intravenous administration of the drug.
|
|


