| Ampalex |
|
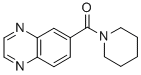
| (CAS 154235-83-3) |
 |
| Description: |
Ampalex (Ampakine CX516; CX516; BDP 12) is an ampakine and nootropic that acts as an AMPA receptor positive allosteric modulator as a treatment for Alzheimer's disease, schizophrenia and mild cognitive impairment (MCI).
IC50 value:
Target: AMPA receptor
Ampalex ameliorates functional deficits in AMPA receptors in a hippocampal slice model of protein accumulation. Researches suggest that AMPA receptors may be potential pharmaceutical targets for the treatment of neurodegenerative diseases, and highlights AMPAkines, in particular, as possible therapeutic agents.
|
| Product No. |
KT20678 |
| Product Name |
Ampalex |
| Synonyms |
|
| Formal Name |
|
| CAS Number |
154235-83-3 |
| Molecular Formula |
C14H15N3O |
| Formula Weight |
241.29 |
| Formulation |
A crystalline solid |
| Purity |
98%min |
| Stability |
2 years |
| Storage |
-20°C |
| Shipping |
USD45 for Europe and USA. No shipping charge once amount reach USD500 |
| Quality Control |
HNMR,CNMR,LCMS,HPLC,IR,etc. |
| Price & Availability |
In Stock. Price Negotiated. |
|
| Related Products: |
Aniracetam Aniracetam(Ro 13-5057) is a nootropics and neuroprotective drug, which is selectively modulates the AMPA receptor and nAChR.
Target: AMPA; nAChR
Aniracetam is an ampakine and nootropic of the racetam chemical class purported to be considerably more potent than piracetam. It selectively modulates the AMPA receptor. It is lipid soluble and has possible cognition enhancing effects. It has been tested in animals extensively, Alzheimer's patients and temporarily-impaired healthy subjects. It has shown potential as an anxiolytic in three clinical animal models [1].
Administration of aniracetam for 10 days (post-natal days (PND) 18-27), at a dose of 50 mg/kg reversed cognitive deficits in both rat genders, indicated by a significant increase in the number of avoidances and the number of 'good learners'. After the termination of the nootropic treatment, a significant increase in both amplitude and frequency of AMPA receptor-mediated mEPSCs in hippocampal CA-1 pyramidal cells was observed [2].
Clinical indications: Cognitive disorder; Stroke
FDA Approved Date:
Toxicity: insomnia; headaches; nausea or rash.
|
|


