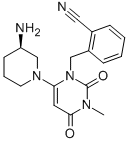
| Alogliptin |
|
| (CAS 850649-61-5) |
 |
| Description: |
Alogliptin(SYR-322) is a potent, selective inhibitor of DPP-4 with IC50 of <10 nM, exhibits greater than 10,000-fold selectivity over DPP-8 and DPP-9.
IC50 value: <10 nM
Target: DPP4
Alogliptin is an orally administered, anti-diabetic drug in the DPP-4 inhibitor class. A randomized clinical trial reporting in 2011 aimed to determine the efficacy and safety of alogliptin versus placebo and voglibose among newly diagnosed Type 2 diabetes patients in Japan. The main outcome indicated that alogliptin was statistically superior to both comparitors. A randomized clinical trial reporting in 2012 aimed to demonstrate that alogliptin was "non-inferior" to a "very low fat/calorie traditional Japanese diet" among newly diagnosed Type 2 diabetes patients in Japan. The outcome indicated that both the drug and dietary treatments comparably impacted indicators of the diabetic condition, such as HbA1c levels and glycemic efficacy. The drug treatment had its impact without changing body mass index (BMI), but the dietary treatment was accompanied by a significant reduction in the BMI…
|
| Product No. |
KT20664 |
| Product Name |
Alogliptin |
| Synonyms |
|
| Formal Name |
|
| CAS Number |
850649-61-5 |
| Molecular Formula |
C18H21N5O2 |
| Formula Weight |
339.397 |
| Formulation |
A crystalline solid |
| Purity |
98%min |
| Stability |
2 years |
| Storage |
-20°C |
| Shipping |
USD45 for Europe and USA. No shipping charge once amount reach USD500 |
| Quality Control |
HNMR,CNMR,LCMS,HPLC,IR,etc. |
| Price & Availability |
In Stock. Price Negotiated. |
|
| Related Products: |
Alogliptin Benzoate Alogliptin benzoate(SYR 322) is a potent, selective inhibitor of DPP-4 with IC50 of <10 nM, exhibits greater than 10,000-fold selectivity over DPP-8 and DPP-9.
IC50 value: <10 nM
Target: DPP4
Alogliptin is an orally administered, anti-diabetic drug in the DPP-4 inhibitor class. A randomized clinical trial reporting in 2011 aimed to determine the efficacy and safety of alogliptin versus placebo and voglibose among newly diagnosed Type 2 diabetes patients in Japan. The main outcome indicated that alogliptin was statistically superior to both comparitors. A randomized clinical trial reporting in 2012 aimed to demonstrate that alogliptin was "non-inferior" to a "very low fat/calorie traditional Japanese diet" among newly diagnosed Type 2 diabetes patients in Japan. The outcome indicated that both the drug and dietary treatments comparably impacted indicators of the diabetic condition, such as HbA1c levels and glycemic efficacy. The drug treatment had its impact without changing body mass index (BMI), but the dietary treatment was accompanied by a significant reduction in the BMI…
|
|


