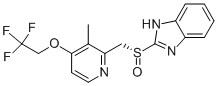
| (R)-Lansoprazole |
|
| (CAS 138530-94-6) |
 |
| Description: |
(R)-Lansoprazole is a proton pump inhibitor which prevents the stomach from producing acid.
Target: Proton Pump
Lansoprazole sodium is sodium salt form of lansoprazole, lansoprazole, a substituted benzimidizole proton pump inhibitor, on pharmacokinetics and metabolism of theophylline has been studied in healthy adults given oral lansoprazole 30 mg once daily for 11 days. On Days 4 and 11 of 300 mg aminophylline was simultaneously administered orally and blood samples for theophylline analysis were taken over 24 h . Patients in the lansoprazole group were significantly less likely to have a recurrence of ulcer complications than patients in the placebo group (P=0.008). There was no significant difference in mortality between the two groups . lansoprazole (AG-1749) and omeprazole, were found to have significant activities against this organism. The activity of lansoprazole was comparable to that of bismuth citrate, with MICs ranging from 3.13 to 12.5 micrograms/ml, and fourfold more potent than that of omeprazole
|
| Product No. |
KT20585 |
| Product Name |
(R)-Lansoprazole |
| Synonyms |
|
| Formal Name |
|
| CAS Number |
138530-94-6 |
| Molecular Formula |
C16H14F3N3O2S |
| Formula Weight |
369.36 |
| Formulation |
A crystalline solid |
| Purity |
98%min |
| Stability |
2 years |
| Storage |
-20°C |
| Shipping |
USD45 for Europe and USA. No shipping charge once amount reach USD500 |
| Quality Control |
HNMR,CNMR,LCMS,HPLC,IR,etc. |
| Price & Availability |
In Stock. Price Negotiated. |
|
| Related Products: |
Esomeprazole magnesium salt Esomeprazole magnesium salt is a proton pump inhibitor which reduces acid secretion through inhibition of the H+ / K+ ATPase in gastric parietal cells.
IC50 value:
Target: proton pump
Esomeprazole sodium (Nexium) is the S-isomer of omeprazole and acts as a proton pump inhibitor and gastric antisecretory agent indicated for the short-term treatment of gastroesophageal reflux disease in patients with a history of erosive esophagitis. Esomeprazole 0.4 and 0.8 mg/mL as the sodium salt in the infusion solutions tested is chemically and physically stable for at least 2 days at room temperature and 5 days under refrigeration.
|
|


