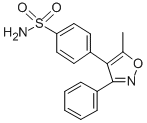
| Valdecoxib |
|
| (CAS 181695-72-7) |
 |
| Description: |
Valdecoxib (SC 65872) is a COX-2 selective inhibitor with an IC50 value of 5 nM.
IC50 Value: 5 nM (COX-2) [1]
Target: COX-2
Valdecoxib is a non-steroidal anti-inflammatory drug (NSAID) used in the treatment of osteoarthritis, rheumatoid arthritis, and painful menstruation and menstrual symptoms.
in vitro: The overall saturation binding affinity for COX-2 of valdecoxib is 2.6 nM (compared with 1.6 nM for celecoxib, 51 nM for rofecoxib, and 260 nM for etoricoxib), with a slow off-rate (t(1/2) approximately 98 min). Valdecoxib inhibits COX-1 in a competitive fashion only at very high concentrations (IC(50) = 150 microM). Collectively, these data provide a mechanistic basis for the potency and in vitro selectivity of valdecoxib for COX-2. Valdecoxib showed similar activity in the human whole-blood COX assay (COX-2 IC(50) = 0.24 microM; COX-1 IC(50) = 21.9 microM) [1]. The affinity of [(3)H]celecoxib for COX-2 (K(D) = 2.3 nM) was similar to the affinity of [(3)H]valdecoxib (K(D) = 3.2 nM). The binding to COX-2 seems to be both rapid and slowly reversible with association rates of 5.8 x 10(6)/M/min and 4.5 x 10(6)/M/min and dissociation rates of 14 x 10(-3)/min (t(1/2) = 50 min) and 7.0 x 10(-3)/min (t(1/2) = 98 min) for [(3)H]celecoxib and [(3)H]valdecoxib, respectively .
in vivo: In rats, valdecoxib demonstrated marked potency in acute and chronic models of inflammation (air pouch ED(50) = 0.06 mg/kg; paw edema ED(50) = 5.9 mg/kg; adjuvant arthritis ED(50) = 0.03 mg/kg). In these same animals, COX-1 was spared at doses greater than 200 mg/kg [1]. Valdecoxib was extensively metabolized after a single 5 mg/kg oral administration of [(14)C]valdecoxib and elimination of unchanged drug was minor (less than 1%) in male and female mice .
Toxicity: Due to a perceived increased risk of thrombotic events, particularly cardiovascular hazards and reports of unpredictable, potentially life threatening skin reactions, valdecoxib has been voluntarily withdrawn from the market since 2005 .
Clinical trial: Valdecoxib in Treating Chronic Pain in Cancer Patients.
|
| Product No. |
KT20583 |
| Product Name |
Valdecoxib |
| Synonyms |
|
| Formal Name |
|
| CAS Number |
181695-72-7 |
| Molecular Formula |
C16H14N2O3S |
| Formula Weight |
314.36 |
| Formulation |
A crystalline solid |
| Purity |
98%min |
| Stability |
2 years |
| Storage |
-20°C |
| Shipping |
USD45 for Europe and USA. No shipping charge once amount reach USD500 |
| Quality Control |
HNMR,CNMR,LCMS,HPLC,IR,etc. |
| Price & Availability |
In Stock. Price Negotiated. |
|
| Related Products: |
(R)-(-)-Ibuprofen (R)-Ibuprofen, a nonsteroidal anti-inflammatory, is the less active enantiomer of ibuprofen, an inhibitor of Cox-1 and Cox-2.
IC50 Value: 121.8uM (inhibited the activation of NF-kB in response to T-cell stimulation) [2].
Target: COX1; COX2
S form ofibuprofen or S(+)-ibuprofen (SIB) is the biologically active isomer and is primarily responsible for the antiinflammatory activity.
in vitro: Cosolvents exponentially increased the solubility of both SIB and racIB, especially in the presence of PG and PEG 300. Glycerol was not very effective in increasing the aqueous solubilities of both compounds, whereas sorbitol solution had a minimal effect on their solubility. PG and PEG 300 increased the solubility of SIB by 400-fold and 1500-fold, respectively, whereas the rise in solubility for racIB was 193-fold and 700-fold, respectively, at 25 degrees C for the highest concentration of the cosolvents used (80% v/v) [1]. When (S)-(+)-IBU and (R)-(-)-IBU (1 microM) were incubated with a panel of recombinant human P450s, only CYP2C9 formed appreciable amounts of the hydroxy metabolites. At a higher IBU enantiomer concentration (500 microM), additional P450s catalyzed 2-hydroxylation (CYP3A4, CYP2C8, CYP2C19, CYP2D6, CYP2E1, and CYP2B6) and 3-hydroxylation (CYP2C19) [3].
in vivo: The presence of the CYP2C8*3 allele was found to influence the pharmacokinetics of (R)-ibuprofen in a gene-dose effect manner. Thus, after administration of 400 mg (R)-ibuprofen, the plasma half-life (95% confidence intervals) for individuals with genotypes CYP2C8*1/*1, CYP2C8*1/*3 and CYP2C8*3/*3, was 2.0 h (1.8-2.2), 4.2 h (1.9-6.5; P < 0.05) and 9.0 h (7.8-10.2; P < 0.002), respectively [2].
Clinical trial: A Study of Aleglitazar in Combination With Ibuprofen in Healthy Volunteers . Phase1
|
|


