| AP24534 |
|
| (CAS 943319-70-8) |
 |
| Description: |
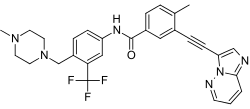
Ponatinib (trade name Iclusig, previously AP24534) is an oral drug developed by ARIAD Pharmaceuticals for the treatment of chronic myeloid leukemia (CML) and Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL). It is a multi-targeted tyrosine-kinase inhibitor.[1] Some forms of CML, those that have the T315I mutation, are resistant to current therapies such as imatinib. Ponatinib has been designed to be effective against these types of tumors.[2]
The United States Food and Drug Administration approved the drug as a candidate in 2012, but temporarily suspended sales on 31 October 2013 because of "the risk of life-threatening blood clots and severe narrowing of blood vessels".[5][6] This suspension was partially lifted on Dec. 20, 2013 with ponatinib being issued revised prescribing information, a new "Black Box Warning" and a "Risk Evaluation and Mitigation Strategy" in place to better evaluate the risks and benefits of using the drug.
|
| Product No. |
KT00085 |
| Product Name |
AP24534 |
| Synonyms |
|
| Formal Name |
|
| CAS Number |
943319-70-8 |
| Molecular Formula |
C29H27F3N6O |
| Formula Weight |
532.56 |
| Formulation |
A crystalline solid |
| Purity |
98%min |
| Stability |
2 years |
| Storage |
-20°C |
| Shipping |
USD45 for Europe and USA. No shipping charge once amount reach USD500 |
| Quality Control |
HNMR,CNMR,LCMS,HPLC,IR,etc. |
| Price & Availability |
In Stock. Price Negotiated. |
|
| Related Products: |
Apatinib
Axitinib
AZD2932
AZD4547
BIBF 1120
BMS-690514
E-3810
|
|


