| GSK1349572 |
|
| (CAS 1051375-16-6) |
 |
| Description: |
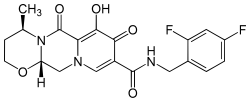
Dolutegravir[2] (DTG) is an FDA-approved drug[3] for the treatment of HIV infection. Dolutegravir is an integrase inhibitor. Known as S/GSK1349572 or just "572" the drug is marketed as Tivicay[4] by GlaxoSmithKline (GSK). In February, 2013 the Food and Drug Administration announced that it would fast track dolutegravir's approval process.[5] On August 13, 2013, dolutegravir was approved by the FDA. On November 4, 2013, dolutegravir was approved by Health Canada.[6] On January 16, 2014, Tivicay was approved by the European Commission for use throughout the European Union.[7]
|
| Product No. |
KT00393 |
| Product Name |
GSK1349572 |
| Synonyms |
|
| Formal Name |
|
| CAS Number |
1051375-16-6 |
| Molecular Formula |
C20H19F2N3O5 |
| Formula Weight |
419.4 |
| Formulation |
A crystalline solid |
| Purity |
98%min |
| Stability |
2 years |
| Storage |
-20°C |
| Shipping |
USD45 for Europe and USA. No shipping charge once amount reach USD500 |
| Quality Control |
HNMR,CNMR,LCMS,HPLC,IR,etc. |
| Price & Availability |
In Stock. Price Negotiated. |
|
| Related Products: |
Abacavir
AMD 3465
Amprenavir
Atazanavir
Atazanavir sulfate
Bevirimat
BMS-378806
BMS-538203
BMS-626529
BMS-663068
BMS-663068 Tris
BMS-707035
|
|


